Abstract
Background: Protein production and homeostasis are critical to life and death decisions of malignant plasma cells in multiple myeloma (MM). While protein homeostasis has been targeted effectively with proteasome inhibitors in this disease, inhibition of protein production has not been clinically tested. Protein translation inhibition represents an immediately available avenue, with the agent omacetaxine mepesuccinate (Oma). Recent in vivo and ex vivo work illustrates the broad sensitivity MM cells have to Oma, irrespective of proteasome inhibitor (PI) or immunomodulatory (IMiD) drug treatment exposure (Walker et al. Clinical Cancer Research, 2021). High levels of baseline protein translation, compared to other cells in the bone marrow, serves as a biomarker of high Oma sensitivity in MM patient samples. Based on this, we hypothesized that Oma treatment in MM may lead to eventual drug resistance through the downregulation of protein synthesis. To test this, we evaluated the effects of long-term Oma treatment on MM cells, by establishing resistance in two human MM cell lines and comparing them to their isogenic parental lines.
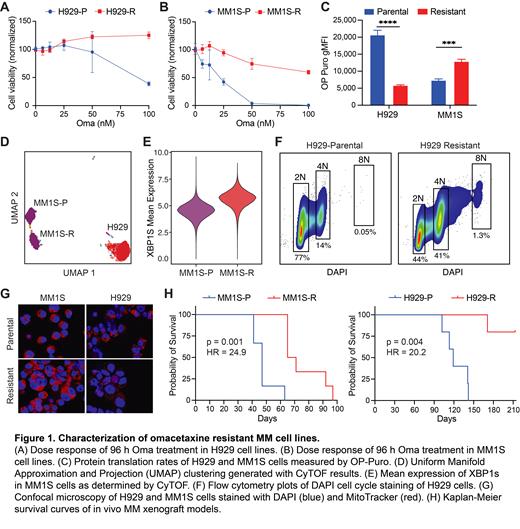
Methods: Omacetaxine resistance was established by dose escalation in MM cell lines NCI-H929 and MM1S, with concentrations gradually increased over 80 weeks to 84 nM and 52 nM, respectively. Resistance was confirmed via CellTiter-Glo after 96 h incubations. Translation rates were measured by incorporation of OP-Puromycin. Flow cytometry was used for cell cycle analysis. Mass cytometry (CyTOF) was performed on a Fluidigm Helios system with a 35-marker panel. Cell morphology was examined by confocal microscopy using a Zeiss LSM780 spectral microscope with DAPI and Mitotracker stains. Cell line xenograft studies were performed using NSG mice engrafted via IV tail injection.
Results: Resistant cell lines H929-R and MM1S-R displayed decreased cytotoxicity to Oma, with EC50 >100 nM after 96 h treatment, compared to parental line sensitivity of 24 nM for MM1S and 54 nM for H929 (Fig 1A and 1B). Next, we evaluated baseline translation rates for both parental and resistant lines. As hypothesized, H929 cells significantly downregulated translation after acquiring Oma resistance, but MM1S cells actually significantly upregulated protein synthesis (n = 3, p < 0.001 for both lines, Fig 1C). Interestingly, MM1S-R cells showed global phenotypic changes from the parental line based on UMAP analysis of CyTOF results, whereas H929-R appeared similar to its isogenic line (Fig 1D). The most altered protein was the transcription factor XBP1s, which was elevated in MM1S-R cells compared to parental (Fig 1E). An increased percentage of aneuploidy cells was observed in both resistant lines compared to the parental lines, indicating genomic pressure on both (Fig 1F). Consistent with this, confocal microscopy showed the emergence of multi-nucleated and pleomorphic cells in the Oma resistant lines compared to parental (Fig 1G). Finally, both resistant lines showed a surprising extended survival in an untreated MM NSG xenograft model. Mice engrafted with either H929-R or MM1S-R had a significant survival advantage over their parental cell lines (p = 0.0019, p = 0.0014, Fig 1G and 1H). Median survival was 119 days for H929-P and unreached for H929-R mice, MM1S was 47 and 68 days respectively.
Conclusion: After prolonged exposure to Oma, cell lines H929 and MM1S displayed unique characteristics compared to their parental lines, including changes in protein translation rates, drug sensitivities, aneuploidy, and in vivo growth characteristics. Once resistant to Oma, H929 cells downregulated translation, similar to published patient sample data. Conversely, MM1S cells upregulated translation and displayed a more complex phenotypic shift, including an upregulation of XPB1s, an indication of increased unfolded protein response. Much like PIs, we believe Oma disrupts the delicate proteostasis intrinsic to MM cells, putting them on the defensive. Both Oma resistant cell lines exhibited a stressed cell state evident through their decline of in vivo fitness and an increase in poly-aneuploidy. Ongoing research with these cell lines will evaluate transcriptional changes, continuing to advance our understanding of Oma sensitivity and resistance in multiple myeloma.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal